Healthcare Trends in the next 5-10 Years and its Potential Impact
COMPANION DIAGNOSTICS, MOLECULAR DIAGNOSTICS, BIOTECH RECRUITER, DIRECT TO CONSUMER TESTING, MEDTECH RECRUITINGThe healthcare and life sciences industry is expected to go through some major shifts in the next 5-10 years, and some of these have already started.
These shifts are linked to five megatrends related to patient behavior, demographic changes, technology adoption, life science innovation, and regulatory paradigm.
First, there is a new level of healthcare consumerism on the horizon. At-home diagnostic devices, smartwatches, and a variety of smartphone apps are already mainstays and are producing a large amount of data. Providers in the yesteryears had held health data; there is an increased demand from patients to access the data so that individuals can better manage their health. There would be increasing data availability levels (clinical, lab, social, economic, and demographic), and patients would truly experience consumerism. This will give birth to new entrants in the healthcare domain, companies with a great understanding of consumer behavior. Large IT companies such as Google/Alphabet, Apple, Amazon, and Microsoft have been very active in healthcare, as tracked by the number of healthcare patents filed between 2013 and 2017.
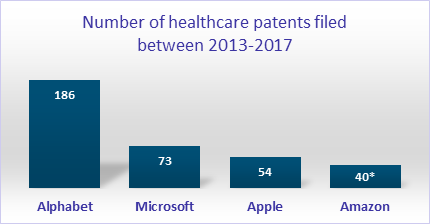
Source: E&Y Report. Amazon # patents estimated
Google/Alphabet has three healthcare subsidiaries, Verily, Calico, and Deepmind, addressing multiple disease areas. Amazon had entered the healthcare space with a joint venture (named Haven) * with Berkshire Hathaway and JP Morgan which would have direct access to 1.2 million employees totaling the three companies, 110 million Amazon Prime customers, and possibly over 310 million Amazon customers. Microsoft is leveraging its AI solution to engage in large strategic partnerships with providers and pharmaceutical companies such as UPMC, UCLA Health, and Novartis. Apple has a partnership with Aetna, a large health insurance company, and another innovative partnership with J&J to incorporate ECG into Apple Watch. These trends are just the beginning and are expected to intensify over the next 5-10 years. A Morgan Stanley report estimates that Apple will have $313 Billion in revenues from the healthcare segment by 2027.
*Haven has announced shutting down its operations by February 2021.
Second, we are in the midst of a demographic shift. With the rise in life expectancy, the number of Americans ages 65 and older is projected to grow from 52 million in 2018 to 73 million by 2030. WHO has projected similar trends for the global population.
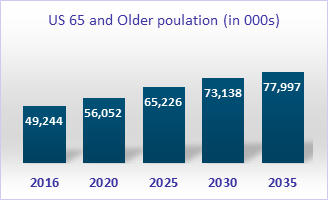 Source: US Census Bureau; The Administration for Community Living
Source: US Census Bureau; The Administration for Community Living
This trend would bring chronic diseases such as diabetes, hypertension, dyslipidemia, obesity, Alzheimer’s, and other behavioral diseases to the forefront. Chronic disease management must find ways to deliver holistic care without burdening the healthcare system. Governments seek low-cost, efficient solutions to reform healthcare and address their depleting resource issues.
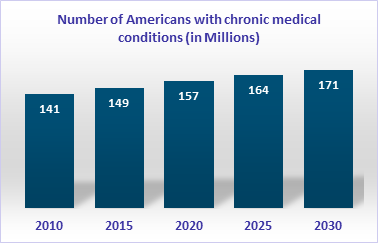
Source: Adapted from Partnership for Solutions. Chronic Conditions: Making the Case for Ongoing Care. Baltimore, MD.
New business models are emerging, with governments collaborating with the private sector to address the situation with transparency and favorable outcomes. The answer seemingly lies in creating new care delivery models to deliver value. We have already seen the advent of ACOs; in 2019 ACOs covered approximately 44 million lives, a 10% increase from 2018. Some experts estimate that ACOs will cover 150 million lives by 2025. Next Generation ACOs have also evolved, and tremendous growth in ACO-like providers is expected in the next five years. Owing to a potential shortage of 45,000 primary care physicians by 2025, the primary care medical home (PCMH) model promises to improve health care in the USA by transforming how primary care is organized and delivered.
“A new analysis shows that ACOs and APMs could save the Centers for Medicare & Medicaid Services (CMS), which runs the Medicare program, $270 million over the next decade.”: National Association of ACOs, Aug. 18, 2020.
Separately, price sensitivity and drug access have given rise to innovative drug reimbursement models. Six important models are emerging: financial risk-based contracts, health outcomes contracts, mortgage, subscription or Netflix models, indication-specific pricing, and volume-based purchasing. Value-based contracts (financial-risk-based contracts or health outcome contracts) can better manage chronic disease drug pricing, for example, blood glucose control in diabetes—measured by HbA1c blood tests—or a reduction in the number of asthma attacks associated with product use. Mortgage models can reduce sensitivity to high-priced products and promote better patient affordability. Products such as new immunotherapy products for oncology, orphan drugs, or emerging gene and cell therapies are good candidates for the mortgage model. These innovative pricing models would further develop in the 5-10 years horizon, and the biopharma industry needs to find the best model for each product.
Third, the healthcare industry is coming around to the adoption of technologies. The adoption of technologies in the healthcare industry has been much slower than in the financial, automotive, aerospace, or consumer goods industries. The use of machine learning, robotics, virtual reality, and automation is finally catching up and will impact life sciences and healthcare significantly in the next 5-10 years. Around 240 start-up companies use AI in drug discovery and development. Even if we consider a specific area of designing and optimizing drug candidates, there are over 110 start-up companies with some significant companies such as Atomwise, AI Therapeutics, BioXcel Therapeutics, Numerate, NuMedii, Relay Therapeutics, and Schrodinger that are making an enormous impact on drug discovery. The AI market in biopharma is expected to grow significantly by 2025.
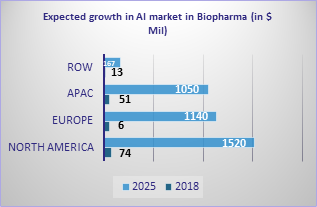 Source: Intelligent Biopharma, Deloitte.
Source: Intelligent Biopharma, Deloitte.
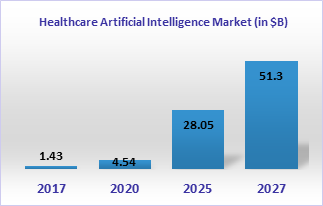 Source: Statista; Meticulous Research®
Source: Statista; Meticulous Research®
Similarly, around 100 startups are applying deep learning in analyzing radiology and pathology images. These technologies would revolutionize the diagnosis of diseases and be fast, accurate, and efficient. Early diagnosis can prevent mortalities from stroke and sepsis. In the next five years, it is expected that these automated diagnostics will be mainstream. Not only nimble MedTech companies but traditional diagnostic companies such as Siemens Healthineers, GE Healthcare, Philips, Canon, and others would also integrate these automated technologies into their imaging equipment.
The adoption of automation would also play a significant role in surgery. Shortly, surgical interventions of all types, from dental implants to deep brain surgery, will be assisted by sophisticated robotic instrumentation and visualization systems, making surgery less invasive, faster, and less expensive. These procedures may even be performed remotely by specialized surgeons while nurses, technicians, and less-trained surgeons attend.
“So navigation is the eyes, robotics is the hand that automates the surgery, and now with AI we have the brain,” Jacob Paul, Senior Vice President of Cranial and Spinal Technologies, Medtronic.
Constituting about 1 million robotic-assisted surgical procedures in the US in 2019, the robotic surgery market is expected to grow impressively and approach 3 million procedures by 2025. Market leaders such as Intuitive Surgical and Accuray are poised to grow with their da Vinci and CyberKnife systems, respectively.
Fourth, life science innovations will deliver therapies for diseases that are hard to treat. The full impact of genomics, proteomics, and metabolomics is now being recognized in precision medicine and chronic diseases. Precision medicine tailored to a patient's unique needs, genetic makeup, and lifestyle will continue to be a significant trend in healthcare. Moving past a one-size-fits-all approach could lead to fewer ineffective interventions and better outcomes. Another area of explosive projected growth is immune-oncology – a promising field of cancer research that coaxes the body’s immune system into fighting the disease. Unlike traditional radiation therapy and chemotherapy approaches, which kill healthy cells and cancerous cells, immunotherapies only target cancer cells.
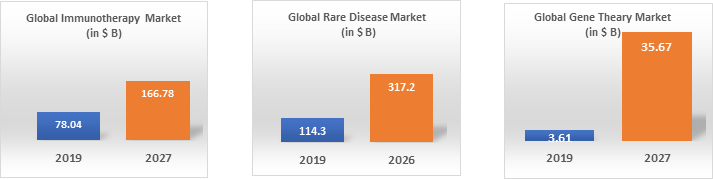
Source: Reprints & Data Source: Global Market Insights Source: Fortune Business Insights
Another burgeoning area in healthcare is gene therapy, which targets a missing or non-functioning gene in a patient’s DNA and adds or replaces it with a working gene that can make a needed protein. While it might seem like a relatively straightforward “potential cure,” we don’t yet know how long the body will respond to gene edits, and we also don’t know how people’s immune systems will react to these interventions over time. There are approximately 7,000 rare diseases affecting an estimated 30 million people in the U.S. Unfortunately; most rare diseases still do not have treatments. In total, the FDA in 2019 approved 76 orphan indications, which included 22 novel drugs and biologics with orphan designation. In 2019, the Office of Orphan Products Development received 533 new requests for orphan disease designation. Interestingly, many of the innovations in immunology, gene therapy, and rare disease therapy are being driven by smaller biotech companies. Big pharma is trying to catch up through strategic partnerships and acquisitions.
Fifth, the US and other international regulatory landscapes are changing. FDA is very intent on making innovative therapies available to patients. New drug approvals by FDA have increased significantly over the last three years. Between 2017 and 2020, an average of 52 new drugs were approved, compared to 32 drugs between 2010 and 2016. Biosimilars and generic approvals are on the rise. Similarly, novel devices approved through original PMAs, panel track supplement PMAs, de novos, HDEs, and breakthrough 510(k)s have more than doubled between 2013 and 2018; 51 approvals in 2013 versus 106 approvals in 2018.
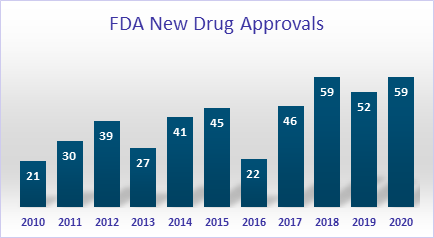
Global clinical trials have supported many of the products approved in the US, and there is greater coordination between regulatory agencies of different countries. At the same time, the FDA is very focused on the safety of drugs and devices. Data transparency has been the cornerstone of promoting the safety of approved products. Drug manufacturers are now voluntarily putting more data in the public domain. FDA has launched a new pilot program to evaluate whether disclosing certain information included within “clinical study reports” (CSRs) following approval improves public access. FDA, in addition, has been working to enhance the efficiency of post-market drug safety surveillance practices. The Cures Act has mandated summary analysis of adverse drug reaction reports for a drug even though the 10,000-individual use threshold is not met. With many new drugs being developed in oncology, and rare and orphan diseases, these drugs will fall under this reporting need. In addition to FDA’s surveillance, the agency increasingly mandates manufacturers to conduct post-marketing studies for drugs and devices. In the next 5-10 years, it is anticipated that drugs and devices will be approved at a record pace, surveillance and post-marketing study requirements will be stricter, and FDA is intent on holding companies accountable for completing these studies on time to ensure product safety and efficacy.
What do all these trends mean for talent development?
The healthcare trends mentioned above would impact talent needs in biotechnology, MedTech, pharma, and medical devices. There are significant opportunities for growth in talent management; however, careful planning and openness to explore new paradigms are critical. Below are some thoughts that might be considered to serve the industry better and prepare the organization's future for growth.
-
Expanding the client/candidate base
-
-
Going beyond serving the talent management needs of traditional biopharma and medical device businesses and building relationships with companies that have an uncanny understanding of the consumers. Healthcare talent management in companies such as Amazon, Google, Microsoft, SalesForce, and Apple should be considered.
-
Expansion of talent base with tech-savvy candidates to address the longer-term trends in using artificial intelligence, automation, and robotics in drug and device development.
-
Develop a significant focus on small biotech companies. Although these companies might have smaller resources than more prominent biopharma companies, innovation in precision medicine, immune-oncology, gene therapy, and rare disease therapy are primarily driven by these biotech companies.
-
-
Expanding the knowledge base of talent management practices
-
Expand knowledge base in value-based healthcare. To address that, familiarity with real-world data and health economics analysis is required. Spotting and attracting talent from related industries with strong analytics backgrounds might be necessary.
-
Developing a talent base in genetics, genomics, and multi-omics would be crucial to innovation for the biopharma industry. This will help in catering to expected growth in precision medicine.
-
A higher focus on talent in therapeutic areas of immune oncology, gene/cell therapy, and rare diseases will make the innovative organizations future-ready. Not only does talent in basic and translational research needs to be managed well, but talent in clinical trials and post-marketing trials operations and management has also increased talent needs. Clinical trials in these therapy areas are vastly different than traditional clinical trial experiences, and talent selection needs to be selective.
-
ABOUT PARTHA PAUL
Partha Paul is the Managing Director of Connexis Search Group and is passionate about developing and motivating talent. Partha has been a biotech/MedTech executive with US and international experience in business and corporate development, strategic planning, revenue generation, and P&L management. Before joining CSG, he headed the North American business for LPIXEL, Inc., an AI-based MedTech company. Before that, he was a Business Unit Head for Philips Health Systems with P&L management responsibility. He also served as Vice President of Strategy & Business Development for GE Healthcare. He previously held senior-level corporate and business development roles in biotech and medical device companies such as AtheroGenics, Inc, and Interleukin genetics, Inc. He started his career as a bench scientist with Southwest Research Institute and focused his research on innovative advanced materials and biomaterials.
ABOUT CONNEXIS SEARCH GROUP
Connexis Search Group is a twenty-year-old permanent placement recruiting firm that serves the following industries: Commercial Laboratory, In-Vitro Diagnostics (IVD), Life Sciences, Biotechnology, and Medical Devices. Our team of over twenty recruiters has industry and functional expertise, enabling us to deliver quality candidates quickly. We are grateful for our success in helping our clients grow their businesses with the best and most qualified candidates.



No Comments